Top stories
Long reads
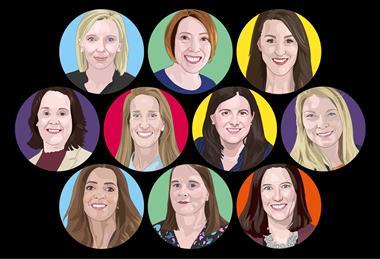
Power list: 10 women at the forefront of grocery technology
Despite a lack of encouragement to study STEM subjects, more and more women are looking to get into tech. Happily, the grocery sector offers plenty of strong, female role models
How long for fmcg until UPFs become next timebomb?
Million customers a month abandoning UPFs, finds Grocer survey
WATCH: Navigating the ultra-processed food debate
Ultra-processed foods: inquiry launches call for evidence on risks
The struggle to process what ultra-processed foods means
Shoppers fear ultra-processed foods bad for their health
What the UPF threat means for fmcg giants
- Previous
- Next
Advert
- Previous
- Next
Already have an account? Sign in here
Sign up to free guest access

Explore our in-depth fmcg intelligence

Sign up to our daily and topic newsletters
People
BP rolls out crime logging platform and body worn cameras for extra staff protection
Power list: 10 women at the forefront of grocery technology
MD Chris Hughes on how Regal Wholesale is on a roll after a major rebrand
Morrisons faces warehouse strike threat
Tesco Aldershot: Grocer 33 store of the week
Parfetts appoints Richard Bone as commercial director
Holland & Barrett pledges to close women’s health ‘gap’ with £4m investment
Harvey Nichols appoints Julia Goddard as new CEO
Waitrose customer service workers no longer at redundancy risk
Ibis Rice’s Nicholas Spencer on Cambodia, dancing and Chris Packham
- Previous
- Next
Advert
- Previous
- Next
Vegan Food Group’s founders on building ‘the vegan Unilever’
Tony’s supply chain guru on changing chocolate sourcing for good
Nathalie Roos: the CEO making black tea cool again
Merchant Gourmet’s Richard Peake on plant-based’s bright future
Peter Batt on driving the ‘Co-op and Nisa success story’
- Previous
- Next
Sponsored content
- Previous
- Next
How can grocery win big during the summer of sport in 2024 and beyond?
WATCH: How can brands and retailers cater to the new empowered consumer?
How fmcg brands can make the most of AI’s potential
WATCH: Navigating the ultra-processed food debate
WATCH: How to crack the online marketplace opportunity
WATCH: How will Brits cook at home in 2024?
WATCH: HFSS one year on: What have we learned?
WATCH: Sustainability vs affordability: how can brands and retailers strike the right balance?
WATCH: How can fmcg create seamless omnichannel experiences?
- Previous
- Next
Advert
- Previous
- Next
Watch and listen
The latest podcasts and videos from The Grocer
Marmite’s latest faux documentary is past its sell-by date
Animal demands attention as Peperami enters ‘the snack wormhole’
Milliways gives plastic gum consumers something to chew on
Greggs bags attention with cheesy quiz show ad
Chocomel chucks everything at random ad campaign
What lies ahead for the Coca-Cola brand in 2024?
Richmond launches £2.6m ‘Good Times’ brand campaign
Tackling crime in convenience: The Convenience Mix Podcast
Kerrygold takes John Lewis route with anthropomorphised table
Just Eat targets Brits with passive-aggressive animations
- Previous
- Next
Guide

SEARCH for the products, services and companies you need in the definitive guide to the UK food and drink industry.
Already have an account? Sign in here
Sign up to free guest access

Explore our in-depth fmcg intelligence

Sign up to our daily and topic newsletters




























































































































![J8865_Group_Shot_V1-2[2]](https://dmrqkbkq8el9i.cloudfront.net/Pictures/380x259/3/5/0/319350_j8865_group_shot_v122_230843.jpg)